IMPORTANT UPDATE
If you are an existing Maze Cord Blood customer and have a billing question or concern, please call 914-683-0000.
As of May 1, 2023, Maze Cord Blood is no longer accepting new enrollments. We’re happy to have helped so many families over the years and will continue to store and care for each baby’s stem cells with the same level of commitment as we always have. If you have any questions about your current account, please contact us.
In our search for a reasonably priced bank with the same level of commitment to service, quality processing, and safe storage, we are happy to announce our partnership with MiracleCord for all new cord blood and cord tissue enrollments. As a leading cord blood bank that has been providing stem cell services for nearly two decades, MiracleCord was included in the Forbes Health “Best Cord Blood Banks of 2023” and was awarded “Best U.S. Cord Blood Bank” by Global Health & Pharma.
For information on cord blood and cord tissue banking, or to enroll, please call our trusted partner MiracleCord at 888-435-5550 or visit their website at MiracleCord.com.
CORD BLOOD BANKING BLOG

Top 5 Ways to Increase Your Milk Supply
Since we can’t measure breast milk intake the way we can formula, it is easy to be insecure about your milk supply. If you feel…

Recommended Workouts During Pregnancy
Worried about potential risks? Don’t be. There just aren’t any if you’ve been green-lighted by your practitioner, you choose the right pregnancy workouts, and you exercise…
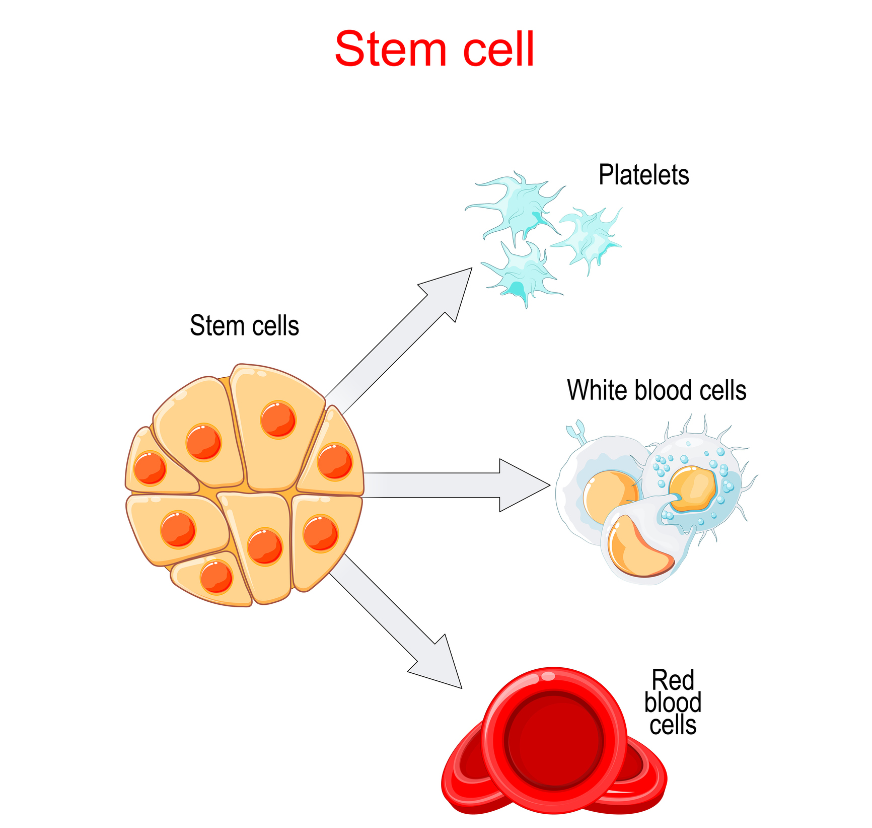
Advancements in Stem Cell Use
It is well known that stem cells found in a newborn baby’s cord blood are rich in (hematopoietic) stem cells, which has been used to…

First Woman Cured of HIV Using Cord Blood Stem Cells
A woman of mixed race becomes the third patient in the world to be cured of HIV, and the first one to do it using…

How has COVID impacted Pregnancy Rates?
Speculation has been as much a part of the COVID pandemic as isolation, tv show reunions, endless deliveries of Chipotle, eye glass fog and Zoom…











